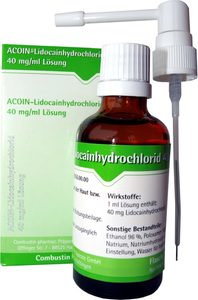
40 mg/ml Solution with attached pump system
The active substance is lidocaine hydrochloride 1 H2O.
1 ml solution contains 40 mg lidocaine hydrochloride (in form of lidocaine hydrochloride 1 H2O).
Further ingredients are: poloxamer 188, saccharin-sodium, sodium hydroxide for PH adjustment, ethanol 96 %.
What exactly is Acoin-Lidocaine Hydrochloride 40 mg/ml Solution and what is it used for?
Acoin-Lidocaine Hydrochloride 40 mg/ml Solution is a drug for local anesthetic.
Acoin-Lidocaine Hydrochloride 40 mg/ml Solution is used for surface anesthesia
Before surgery and diagnostic interventions in throats and noses
When tissue samples are taken (biopsy) from the oral cavity and nasopharyngeal cavity
Before endoscopy of the trachea or lung (bronchoscopy)
Before X-ray examination of the upper respiratory tract, after injection of contrast agents (bronchography).
Type of application
For application in the mouth and throat region. This product is not suitable for injection purposes.
Acoin-Lidocaine Hydrochloride 40 mg/ml Solution must not be applied in the following cases:
with a previously damaged mucosa (if you are not sure please contact your doctor).
Possible side effects
Reporting side effects
If you notice any side-effects you should tell your doctor or pharmacist. This applies also for those side-effects that are not described in this leaflet. You can report side-effects directly to the Bundesinstitut für Arzneimittel und Medizinprodukte, (the Federal Institute for Drugs and Medical Devices), Abt. Pharmakovigilanz,
Kurt-Georg-Kiesinger-Allee 3, D53175 Bonn, Germany. Website: www.bform.de.
By reporting the side-effects, you can contribute to gathering more information on the safety of this medical product.
Storage conditions for Acoin-Lidocaine Hydrochloride 40 mg/ml Solution
Always store pharmaceuticals out of reach of children.
Do not use the product after the expiry date which is stated on the label/carton.
Shelf-life after opening: 3 months
Please get in touch via international@combustin.de if you are interested in receiving more information about our product highlights.